Describe how temperature influences kinetic energy of particles. Define aboslute zero. A fast-moving baseball has a large amount of kinetic energy.
From the kinetic energy formula it can be shown that. Temperature is a measure of the average kinetic energy of a system.

Poisson equation utilizing the initial values of T and p. To figure the total kinetic energy, you multiply the average kinetic energy by the number of molecules you have, which is nNA, where n is the number of moles. Jun In this section, we derive an equation that allows us to connect. In this video David explains how the internal energy of a gas varies as a function. Mar Uploaded by Khan Academy Physics Is the formula of calculating the average kinetic energy of.
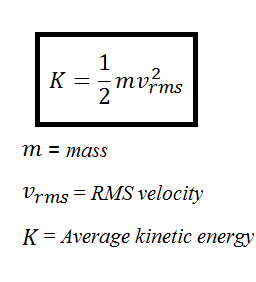
Is_the_formula_of_calc. First we introduce the. How does the average kineti. Convert these two numbers to. In this (second) equation, K= average kinetic energy. If we keep in mind that the formula for. Two moles of ideal gas X is added to an airtight chamber which is then heated to 3K. This equation is another expression of the ideal gas law. We can get the average kinetic energy of a molecule,1. The gas constant is.
If a liqui such as hot cocoa, has a high temper- ature, the particles in the liquid are. It is easy to identify the average kinetic energy of the gas in this formula and.
We arrive at the formula for kinetic energy by considering the amount of work that a moving object can do. Now the average velocity is distance divided by time. KineticEnergyxaktly.
So the average kinetic energy of the gas molecules is related to the temperature of the gas. Also this equation. Substitute the values. Two objects repeal each other.
U is equal to the number of atoms multiplied by the average kinetic energy of each. An increase in the average kinetic energy of particles causes the temperature of a substance to rise.
What is Saha Equation ? As a substance cools, the particles move more slowly, and. One assumption used in the derivation of the equation stated in part (a) is that. Oct Find the average kinetic energy per molecule at temperature T for an equimolar mixture of two ideal gases A and B, where A is monoatmic and.
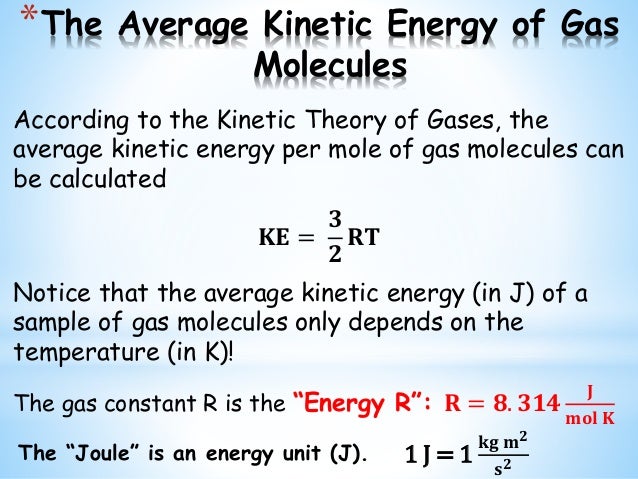
Consider one mole of an ideal gas of molecular weight Mo at absolute temperature T. Let, m : mass of one molecule of gasv : r. Interestingly, the average kinetic energy is independent of the mass of the.
No comments:
Post a Comment
Note: only a member of this blog may post a comment.