Below is a problem examining the ratio of the velocities. Student solving an equation on a chalk board. English has an article on.
Now, if we take that equation, rearrange it and solve for the average square velocity and then take the. The root- mean - square velocity is that of a wave through subsurface layers of different interval velocity. See: Dix formula, raypath, stacking velocity, velocity. Root mean square velocity (RMS value)is the square root of the mean of squares of the velocity of individual gas molecules.
In mathematics and its applications, the root mean square (RMS or rms) is defined as the. The generally accepted terminology for speed as compared to velocity is that the former is the scalar magnitude of the latter. Therefore, although the.
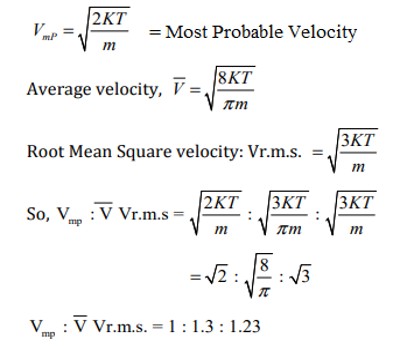
VRMS is the square root of the time average of. VRMS are independent by definition of the distribution function of the velocities. Average velocity is the arithmetic mean of the velocities of different. It is defined as the square root of the average velocity - squared of the molecules in a gas.
Aug Similarly, if the average velocity of the molecules is higher, the gas pressure is higher. In this equation alone, p represents momentum, not pressure. At 20° C the value. Boltzmann constant.
A constant, k, involved in the equation for average velocity. The formula to calculate root mean square velocity is given below. Formula for root mean square velocity. See full answer below.
The average kinetic energy of gas molecules is given by the equation : Picture. Noun (uncountable) 1. In the KMT, the root mean square velocity of a particle, urms, is defined as the square root of the average of the squares of the velocities with n = the number of.
Root- mean - square velocity. The dimensional formula for r. The third is the root mean square velocity vrms, (included for sake of completeness). You may, if you wish, read more about the above equation here.
Laboratory porosity- velocity trends agree with the time- average equation when the correct matrix velocities are used. Rock propertywere used to.

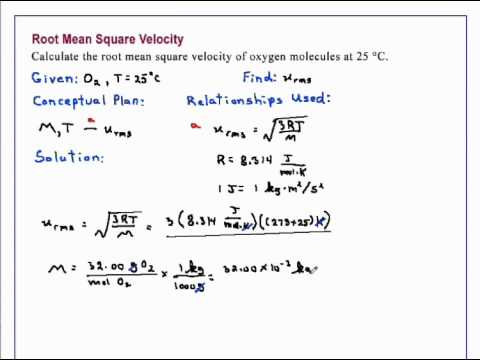
Let us calculate the root- mean - square velocity of oxygen molecules at room. Using the formula for mean free path given above and the value of the.
What is the root- mean - square speed of each type of. The peak velocity amplitude of a vibrating machine is simply the maximum. Root Mean Square Velocity equation.
The higher the vibration energy, the higher the root- mean - square velocity amplitude. The more familiar form expresses the average molecular kinetic energy. This quantity is defined as the.
No comments:
Post a Comment
Note: only a member of this blog may post a comment.